Overview
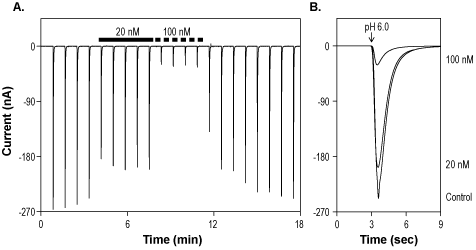 Alomone Labs Mambalgin-1 inhibits ASIC1a channels expressed in Xenopus oocytes.A. Representative time course of Mambalgin-1 (#STM-100) inhibition of ASIC1a. Currents were elicited every 50 sec by transient application of pH 6, while membrane potential was held at -80 mV, and inhibited by 20 nM and 100 nM Mambalgin-1 (indicated by bars). B. Superimposed traces of ASIC1a currents upon application of control, 20 nM and 100 nM Mambalgin-1 (taken from the recording in A).
Alomone Labs Mambalgin-1 inhibits ASIC1a channels expressed in Xenopus oocytes.A. Representative time course of Mambalgin-1 (#STM-100) inhibition of ASIC1a. Currents were elicited every 50 sec by transient application of pH 6, while membrane potential was held at -80 mV, and inhibited by 20 nM and 100 nM Mambalgin-1 (indicated by bars). B. Superimposed traces of ASIC1a currents upon application of control, 20 nM and 100 nM Mambalgin-1 (taken from the recording in A).
- Diochot, S. et al. (2012) Nature 490, 552.
- Mourier, G. et al. (2016) J. Biol. Chem. 291, 2616.
Mambalgin-1 is a 57-residue peptide toxin originally isolated from the black mamba snake venom. It is a selective and reversible acid-sensing ion channel 1 (ASIC1) channel blocker. It binds to the closed state of the channel. The binding induces strong shift of the pH-dependent activation of ASIC1 channel towards a more acidic pH. Mambalgin-1 inhibits homomeric rat ASIC1a, rat ASIC1b, and heteromeric rat ASIC1a + ASIC2a channels heterologously expressed in Xenopus oocytes with an IC50 values of 3.4 ± 0.6, 22.2 ± 1.7, and 152 ± 21 nM, respectively2.
In mice, Mambalgin-1 is not toxic, but poses analgesic effects upon central and peripheral injection found to be as strong as morphine1,2.
ASIC channels are proton-gated Na+ channels directly activated by extracellular protons. They are expressed throughout the CNS and the peripheral nervous system. The channels are involved in a variety of physiological and pathophysiological processes including synaptic plasticity, neuronal injury, nociception and mechanoperception2.
Mambalgin-1 (#STM-100) is a highly pure, synthetic, and biologically active peptide toxin.

