Overview
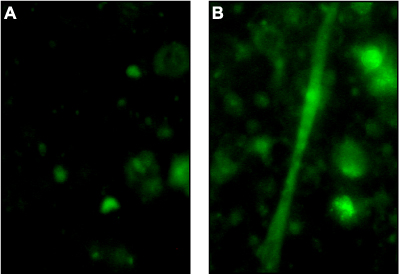 Alomone Labs Maurocalcine evokes long lasting Ca2+ release events in skeletal myotubes.Myotube cell cultures were prepared from limbs of 2 days old rat pups, were loaded with 4 mM fluo-3 AM and then stimulated with Maurocalcine (#RTM-100). Ca2+ spreads in the same myotube before (A) and after (B) stimulation with 200 nM Maurocalcine.
Alomone Labs Maurocalcine evokes long lasting Ca2+ release events in skeletal myotubes.Myotube cell cultures were prepared from limbs of 2 days old rat pups, were loaded with 4 mM fluo-3 AM and then stimulated with Maurocalcine (#RTM-100). Ca2+ spreads in the same myotube before (A) and after (B) stimulation with 200 nM Maurocalcine. Alomone Labs Maurocalcine prolongs the duration of spontaneous long lasting Ca2+ release events in cardiomyocytes.High density cardiomyocyte cell culture prepared from hearts of 3-4 day old rat pups were used 3 days after seeding. The cells were loaded with 4 mM fluo-3 AM and the Ca2+ traces were measured before (blue) and after (red) stimulation with 400 nM Maurocalcine (#RTM-100).
Alomone Labs Maurocalcine prolongs the duration of spontaneous long lasting Ca2+ release events in cardiomyocytes.High density cardiomyocyte cell culture prepared from hearts of 3-4 day old rat pups were used 3 days after seeding. The cells were loaded with 4 mM fluo-3 AM and the Ca2+ traces were measured before (blue) and after (red) stimulation with 400 nM Maurocalcine (#RTM-100).
Maurocalcine (MCa) is a peptidyl toxin originally isolated from the venom of the scorpion Scorpio maurus palmatus.
Native MCa is present at a low concentration in the venom (0.5% of total protein). This 33-mer basic peptide contains three disulfide bridges and has 82% sequence homology to Imperatoxin A (IpTxa), a scorpion toxin from the venom of Pandinus imperator. MCa shares the highly basic structural domain identified in peptide A - a segment of the dihydropyridine receptor and IpTxa which is important for interactions with ryanodine receptors. MCa also appears to share the same features as the so-called cell-penetrating peptides which allows the toxin to efficiently penetrate various cell types without expending metabolic energy or using an endocytotic mechanism.1-4
In vitro, electrophysiological experiments based on recordings of single channels incorporated into planar lipid bilayers showed that MCa potently and reversibly modifies channel gating behavior of the ryanodine receptor 1 (RyR1) by inducing prominent subconductance behavior.1,2
Similar to IpTxa, MCa-modified channels can reversibly shuttle between subconductance states and fast gating states. The actions of these peptides were also found to be additive with those of ryanodine, resulting in additional sub-states along with the ryanodine-locked half-conducting state. However, the predominant subconductance induced by MCa differs from that of IpTxa. At a holding potential of +40 mV, the predominant substates induced by sMCa and IpTxa are 48 and 28% of the full conductance, respectively, suggesting that the structural difference in these peptides may induce slightly different channel conformations that stabilize different unitary conductances.5

