Overview
- Bradshaw, R.A. (1978) Annu. Rev. Biochem 47, 191.
- Bax, B. et al. (1997) Structure 5, 1275.
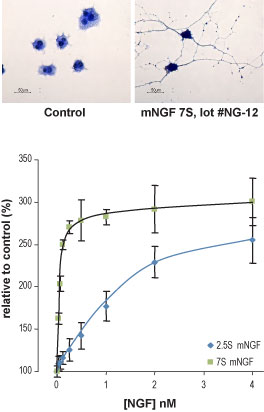 Alomone Labs Native mouse NGF 7S protein promotes neurite outgrowth and survival in PC12 cells.Upper panel: Cells were grown on collagen-coated plates in the absence or presence of 5 ng/ml Native mouse NGF 7S protein (#N-130). The development of neurites over a period of 3 days was visualized by Methylene Blue staining. Lower panel: Cells were grown in the absence of serum and in the presence of varying concentrations of Native mouse NGF 7S protein or Native mouse NGF 2.5S protein (>95%) (#N-100). Cell survival after 24 h was measured by the XTT method and calculated as a relative percentage of the control and plotted against concentrations used.
Alomone Labs Native mouse NGF 7S protein promotes neurite outgrowth and survival in PC12 cells.Upper panel: Cells were grown on collagen-coated plates in the absence or presence of 5 ng/ml Native mouse NGF 7S protein (#N-130). The development of neurites over a period of 3 days was visualized by Methylene Blue staining. Lower panel: Cells were grown in the absence of serum and in the presence of varying concentrations of Native mouse NGF 7S protein or Native mouse NGF 2.5S protein (>95%) (#N-100). Cell survival after 24 h was measured by the XTT method and calculated as a relative percentage of the control and plotted against concentrations used.
The neurotrophins ("neuro" means nerve and "trophe" means nutrient) are a family of soluble, basic growth factors which regulate neuronal development, maintenance, survival and death in the CNS and PNS.1
NGF, the first member of the family to be discovered, was originally purified as a factor able to support survival of sympathetic and sensory spinal neurons in culture.2 It is synthesized and secreted by sympathetic and sensory target organs and provides trophic support to neurons as they reach their final target.3
Neurotrophin secretion increases in the nervous system following injury. Schwann cells, fibroblasts, and activated mast cells normally synthesize NGF constitutively, however direct trauma and induction of cytokines combine to increase neurotrophin production in these cells after injury.4
NGF is purified in three forms: the 7S, 2.5S and β. The 7S (130 kDa) form occurs naturally in mouse submaxillary glands, and is a multimeric protein composed of two α, one β and two γ subunits. The name is derived from its sedimentation co-efficient, 7S.
The biologically active subunit is the β, which is a 26 kDa dimer composed of two identical 120 amino acid chains held together by hydrophobic interactions.5 The α chain stabilizes the 7S complex and binds two zinc ions per 7S complex as a cofactor at the β-γ interface. The γ chain is an arginine specific protease, it may also have plasminogen activator activity as well as mitogenic activity for chick embryo fibroblasts.6 The 2.5S form is 9 amino acids shorter than the β form because of proteolysis that occurs during the purification process.7
The structural hallmark of all the neurotrophins is the characteristic arrangement of the disulfide bridges known as the cysteine knot, which has been found in other growth factors such as Platelet-derived growth factor.8 There is a 95.8% homology between the rat and mouse forms, and a 85% homology between the human and mouse.
NGF has been shown to regulate neuronal survival, development function and plasticity.9 Recently, involvement of NGF in processes not involving neuronal cells has been shown, such as asthma,10 psoriasis11 and wound healing.12 The biological effects of NGF are mediated by two receptors: TrkA, which is specific for NGF, and p75NTR, which binds all the neurotrophins.13
