Overview
- Koike, M. et al. (1997) Neurosci. Res. 29, 27.
- Koike, M. et al. (1997) Neurosci. Res. 29, 27.
1-Naphthyl acetyl spermine (NASPM), a synthetic analogue of Joro spider toxin (JSTX), is a selective antagonist of Ca2+-permeable AMPA receptors. It blocks AMPA receptors lacking the GluR2 subunit expressed in rat hippocampal neurons with IC50 values of 0.33 μM1.
NASPM has been shown to block excitatory synaptic potentials evoked by stimulation of parallel fibers in guinea pig cerebellar Purkinje cells. In the hippocampus, NASPM has been reported to exert a potent and selective suppression of hippocampal epileptic discharges mediated by non-NMDA receptors. In addition, it has been reported that EPSCs in CA1 neurons of the gerbil hippocampus after ischemia are mediated by Ca2+-permeable non-NMDA receptors, and that these abnormal EPSCs are effectively suppressed by NASPM.
These data suggest that NASPM may be useful as a pharmacological tool for investigating the physiological and pathological roles of AMPA receptors, and furthermore, that it could be used as a parent compound to develop new agents for the treatment of neuronal cell death and epilepsy1.
Naspm trihydrochloride (#N-215) is a highly pure, synthetic, and biologically active compound.
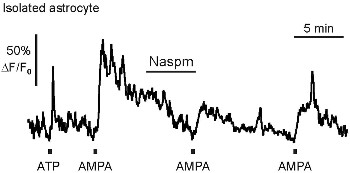
Alomone Labs Naspm trihydrochloride reduces AMPA receptor-induced Ca2+ transients in isolated olfactory bulb astrocyte.Isolated mouse olfactory bulb astrocyte was treated with Naspm trihydrochloride (#N-215). On average, 25 μM AMPA induced Ca2+ transients with an amplitude of 2.50 ± 0.50 ΔF/F0 (n = 31), which were reduced to 52.7 ± 5.3% by Naspm trihydrochloride.Adapted from Droste, D. et al. (2017) Sci. Rep. 7, 44817. with permission of Nature publishing group.
Applications
Citations
- Mouse olfactory bulb (whole-cell voltage clamp).
Beiersdorfer, A. and Lohr, C. (2019) Front. Cell. Neurosci. 13, 451.
- Droste, D. et al. (2017) Sci. Rep. 7, 44817.


