Overview
- Gomez-Lagunas, F. et al. (1996) J. Memb. Biol. 152, 49.
- Tenenholz, T.C. et al. (1997) Biochemistry 36, 2763.
- Rogowski, R.S. et al. (1996) Mol. Pharmacol. 50, 1167.
- Whyment, A.D. et al. (2011) Neuroscience 178, 68.
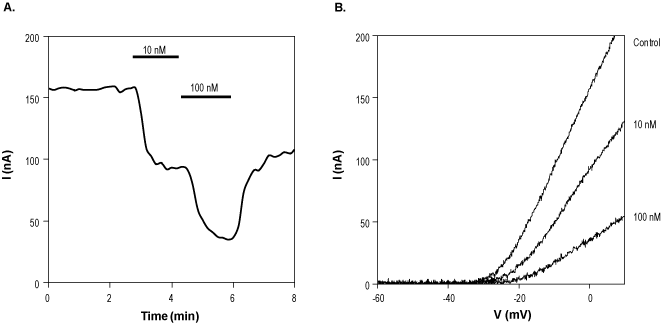 Alomone Labs Pandinotoxin Kα inhibits KV1.2 channels heterologously expressed in Xenopus oocytes.A. Time course of Pandinotoxin Kα (#STP-500) action on KV1.2 currents. KV1.2 currents were elicited by 100 ms voltage ramp from the holding potential of -80 mV to +10 mV. 10 nM and 100 nM Pandinotoxin Kα were perfused as indicated by the bars at -10 mV. B. Superimposed examples of KV1.2 channel current in the absence (control) and presence of 10 nM or 100 nM Pandinotoxin Kα (taken from the experiment in A).
Alomone Labs Pandinotoxin Kα inhibits KV1.2 channels heterologously expressed in Xenopus oocytes.A. Time course of Pandinotoxin Kα (#STP-500) action on KV1.2 currents. KV1.2 currents were elicited by 100 ms voltage ramp from the holding potential of -80 mV to +10 mV. 10 nM and 100 nM Pandinotoxin Kα were perfused as indicated by the bars at -10 mV. B. Superimposed examples of KV1.2 channel current in the absence (control) and presence of 10 nM or 100 nM Pandinotoxin Kα (taken from the experiment in A).
Scorpion venoms are a rich source of ion channel modulators. The scorpion toxins have been useful molecules in probing the K+ channel structures and functions1.
Pandinotoxin Kα (PiTx-Kα) is a 35 amino acid peptidyl toxin isolated from the Pandinus imperator (Emperor scorpion) venom. It is a highly potent and selective blocker of voltage-activated K+ channels. PiTx-Kα preferentially blocks rapidly inactivating (A-type) K+ channels2. In general, channel inhibitors can be pore blockers or gating modifiers. Pore blockers bind to the channel in 1:1 stoichiometry and plug the pore of the channel impeding the flow of the ionic current. These toxins are small proteins that block the passage of K+ ions by binding at the pore entryway on the extracellular side of the channel, thereby inhibiting the ion flux3. The interactions of toxins with K+ channels are among the strongest and most specific known in protein-protein complexes4.

