Overview
- McCormack, K. et al. (2013) Proc. Natl. Acad. Sci. U. S. A. 110, E2724.
 Alomone Labs PF-04856264 blocks NaV1.7 channels expressed in Xenopus oocytes.A. Time course of NaV1.7 current amplitude and inhibition by 500 nM PF-04856264 (#P-265). Currents were elicited by application of a 100 ms voltage step to 0 mV (H.P= -70mV, to induce inactivation), every 10 seconds. B. Superimposed example traces of current responses before and during perfusion of 500 nM PF-04856264, as indicated.
Alomone Labs PF-04856264 blocks NaV1.7 channels expressed in Xenopus oocytes.A. Time course of NaV1.7 current amplitude and inhibition by 500 nM PF-04856264 (#P-265). Currents were elicited by application of a 100 ms voltage step to 0 mV (H.P= -70mV, to induce inactivation), every 10 seconds. B. Superimposed example traces of current responses before and during perfusion of 500 nM PF-04856264, as indicated.
- McCormack, K. et al. (2013) Proc. Natl. Acad. Sci. U. S. A. 110, E2724.
Voltage-gated sodium (NaV) channels contribute to physiological and pathophysiological electrical signaling in nerve and muscle cells. The NaV1.7 is highly expressed on the exons somatic afferent neurons and is thought to play an important role in the signaling of inflammatory pain.
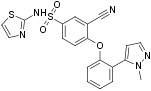
PF-04856264, is a synthetic blocker of the human NaV1.7 channel. It has an effective concentration of 10 nM - 1 μM and an IC50 of 28 nM for NaV1.7 channel in its inactive state. NaV1.7 currents elicited by 20 ms depolarizing voltage steps from −120 mV are insensitive to inhibition by 1 µM PF-04856264, whereas the same concentration produced 91% ± 4% inhibition when a test pulse was preceded by an 8-s conditioning voltage step to inactivate approximately half of the channels. In contrast, NaV1.3 and NaV1.5 channels exhibit little or no sensitivity to inhibition by PF-04856264. Interestingly, different species orthologs exhibit different sensitivities to inhibition by PF. PF interacts with domain 4 voltage sensor M1,2,3 residues of the channel. When these extracellular residues are substituted with residues from the NaV1.3 channel the blocking effect of PF is abolished. Given the involvement of NaV in a variety of pathological conditions further research of subtype selective NaV channel inhibitors may prove useful for developing future therapeutic agents.
PF-04856264 (#P-265) is a highly pure, synthetic, and biologically active compound.
Applications
Citations
- Tompkins, J.D. et al. (2016) Am. J. Physiol. 311, C643.