Overview
- Gunthorpe, M.J. et al. (2007) J. Pharmacol. Exp. Ther. 321, 1183.
- Kort, M.E. and Kym, P.R. (2012) Prog. Med. Chem. 51, 57.
- Szallasi, A. et al. (2007) Nat. Rev. Drug Discov. 6, 357.
- Conway, S.J. (2008) Chem. Soc. Rev. 37, 1530.
- Holland, C. et al. (2014) Br. J. Clin. Pharmacol. 77, 777.
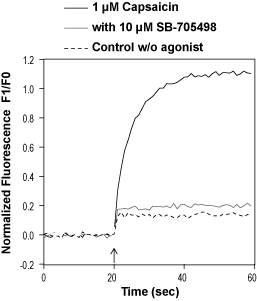 Alomone Labs SB-705498 inhibits TRPV1 channels heterologously expressed in C-6 cells.Cells were loaded with Fluo-3 AM and changes in intracellular Ca2+ were detected. Normalized fluorescence of control cells (untreated and unstimulated), cells responding to 1µM Capsaicin (#C-125), with or without pre-incubation with 10 µM SB-705498 (#S-160), as indicated.
Alomone Labs SB-705498 inhibits TRPV1 channels heterologously expressed in C-6 cells.Cells were loaded with Fluo-3 AM and changes in intracellular Ca2+ were detected. Normalized fluorescence of control cells (untreated and unstimulated), cells responding to 1µM Capsaicin (#C-125), with or without pre-incubation with 10 µM SB-705498 (#S-160), as indicated.
- Kort, M.E. and Kym, P.R. (2012) Prog. Med. Chem. 51, 57.
- Szallasi, A. et al. (2007) Nat. Rev. Drug Discov. 6, 357.
- Conway, S.J. (2008) Chem. Soc. Rev. 37, 1530.
- Holland, C. et al. (2014) Br. J. Clin. Pharmacol. 77, 777.
SB-705498 is a specific and potent antagonist for TRPV1. Generally, TRPV1 inhibitors are classified as either both capsaicin and proton blockers, and mainly capsaicin blockers. SB-705498 belongs to the first class2. After experimenting with lesser potent and effective antagonists, the major advantageous modification the compound's developers had introduced into the related TRPV1 archetypal inhibitors was a trifluoromethyl-substituted pyridine ring; it presented SB-705498 with good solubility and oral bioavailability, superior pharmacokinetic properties1-3.
SB-705498 was developed as an intranasal medication for the treatment of neuropathic and inflammatory pain. Recent clinical trials checked the effectiveness of the compound in treating rhinitis, alleviating dental pain, and relieving acute migraine headache2,4.
SB-705498 (#S-160) is a highly pure, synthetic, and biologically active compound.
Applications
Citations
- Tanaka, Y. et al. (2016) Neuron 90, 1215.

