Overview
- Jin, W. and Lu, Z. (1998) Biochemistry 37, 13291.
- Drici, M.D. et al. (2000) Br. J. Pharmacol. 131, 569.
- Kitamura, H. et al. (2000) J. Pharmacol. Exp. Ther. 293, 196.
- Jin, W. et al. (1999) Biochemistry 38, 14294.
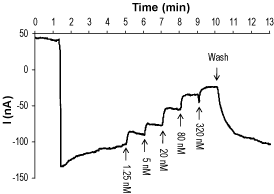 Alomone Labs Tertiapin-Q inhibits Kir3.2 channel heterologously expressed in Xenopus oocytes.A continuous current trace recorded at a holding potential of -80 mV. Kir3.2 currents are downward reflections activated by high K+ containing solution. While activated, increasing concentrations of Tertiapin-Q (#STT-170) were applied (arrows at the bottom of the trace).
Alomone Labs Tertiapin-Q inhibits Kir3.2 channel heterologously expressed in Xenopus oocytes.A continuous current trace recorded at a holding potential of -80 mV. Kir3.2 currents are downward reflections activated by high K+ containing solution. While activated, increasing concentrations of Tertiapin-Q (#STT-170) were applied (arrows at the bottom of the trace). Alomone Labs Tertiapin-Q blocks Kir1.1 channels expressed in Xenopus oocytes.Representative time course of Kir1.1 current, stimulated by a continuous application (top dotted line) of high K+ concentration, and reversible inhibition by 5 nM and 50 nM Tertiapin-Q (#STT-170), as indicated (horizontal bar), at a holding potential of -80 mV.
Alomone Labs Tertiapin-Q blocks Kir1.1 channels expressed in Xenopus oocytes.Representative time course of Kir1.1 current, stimulated by a continuous application (top dotted line) of high K+ concentration, and reversible inhibition by 5 nM and 50 nM Tertiapin-Q (#STT-170), as indicated (horizontal bar), at a holding potential of -80 mV.
- Jin, W. and Lu, Z. (1998) Biochemistry 37, 13291.
- Drici, M.D. et al. (2000) Br. J. Pharmacol. 131, 569.
- Kitamura, H. et al. (2000) J. Pharmacol. Exp. Ther. 293, 196.
- Peleg, S. et al. (2002) Neuron 33, 87.
- Kanjhan, R. et al. (2005) J. Pharmacol. Exp. Ther. 314, 1353.
Tertiapin, the native toxin, was originally isolated from European honey bee Apis mellifera venom. Native and synthetic Tertiapin blocks a range of inward rectifier K+ channels (Kir), in particular ROMK1 (Kir1.1, IC50 = 2 nM) and GIRK (Kir3 family, IC50 for the Kir3.1/3.4 heteromer was 8.6 nM) but with no effect on the Kir2 family member1. In accordance, it was shown to inhibit acetylcholine induced K+ currents in mammalian cardiomyocytes2,3.
Tertiapin-Q is a derivative of Tertiapin in which Met13 is substituted by a Gln residue. However, unlike native Tertiapin, Tertiapin-Q is non-oxidizable and therefore is more stable4.
Tertiapin-Q inhibits the above-mentioned channels with similar affinities and also inhibits Ca2-activated large conductance BK-type K+ channels in a concentration and voltage-dependent manner5.
Tertiapin-Q (#STT-170) is a highly pure, synthetic, and biologically active peptide toxin.
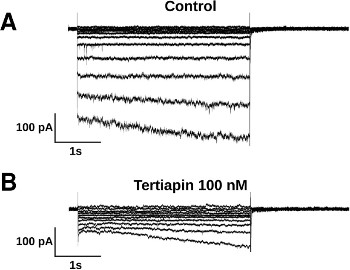
Alomone Labs Tertiapin-Q blocks time-dependent hyperpolarization-activated current (IKH) in rat atrial cardiomyocytes.Whole-cell voltage-clamp was performed on neonatal rat atrial cardiomyocyte (NRAM) primary cultures. A. The voltage-clamp recordings depict large inward currents evoked by hyperpolarizations (5 sec), whereas, very little current is activated during steps to -50 mV and more positive potentials. Na+ current (INa) and T-type Ca2+ current (ICaT) are inactivated by holding the cells at the potential of -40 mV. B. In the presence of Tertiapin-Q (#STT-170) (100 nM), which selectively suppresses the constitutively active acetylcholine (ACh)-mediated K+ current IKACh-c component of IKH, only inward rectifier current (IK1) is left with K+ leak.Adapted from Majumder, R. et al. (2016) PLoS Comput. Biol. 12, e1004946. with permission of PLoS.
Applications
Citations
 Li+ modulates GIRK activity in hippocampal neurons.Incubation of neurons with 1 mM Li+ increased Ibasal in low-K+ solution. Ibasal was measured at −120 mV (voltage step from the holding potential of −60 mV) to enhance the sensitivity of measurement. Representative traces recorded in the low-K+ solution before (black) and after (pink) application of Tertiapin-Q (#STT-170).
Li+ modulates GIRK activity in hippocampal neurons.Incubation of neurons with 1 mM Li+ increased Ibasal in low-K+ solution. Ibasal was measured at −120 mV (voltage step from the holding potential of −60 mV) to enhance the sensitivity of measurement. Representative traces recorded in the low-K+ solution before (black) and after (pink) application of Tertiapin-Q (#STT-170).
Adapted from Farhy Tselnicker, I. et al. (2014) with permission of Proceedings of the National Academy of Sciences.
- Gunther, T. et al. (2016) Mol. Endocrinol. 30, 479.
- Li, Y. et al. (2016) PLoS ONE 11, e0155006.
- Majumder, R. et al. (2016) PLoS Comput. Biol. 12, e1004946.
- Hablitz, L.M. et al. (2015) J. Neurosci. 35, 14957.
- Kotecki, L. et al. (2015) J. Neurosci. 35, 7131.
- Meneses, D. et al. (2015) Synapse. 69, 446.
- Farhy Tselnicker, I. et al. (2014) Proc. Natl. Acad. Sci. U.S.A. 111, 5018.
- Nakamura, A. et al. (2014) Br. J. Pharmacol. 171, 253.
- Kanbara, T. et al. (2014) Neurosci. Lett. 580, 119.
- Nockemann, D. et al. (2013) EMBO Mol. Med. 5, 1263.
- Kailey, B. et al. (2012) Am. J. Physiol. 303, E1107.
- Jo, H.Y. et al. (2011) Biochem. Biophys. Res. Commun. 407, 687.
- Walsh, K.B. (2011) Front. Pharmacol. 2, 1.
- Arora, D. et al. (2010) J. Neurochem. 114, 1487.
- Clark, B.D. et al. (2009) J. Neurosci. 29, 11237.
- Young, C.C. et al. (2009) J. Physiol. 587, 4213.

