Overview
- Meir, A. et al. (2011) Novel peptides isolated from spider venom, and uses thereof. U.S. Patent Application Publication # US 2011/0065647 A1.
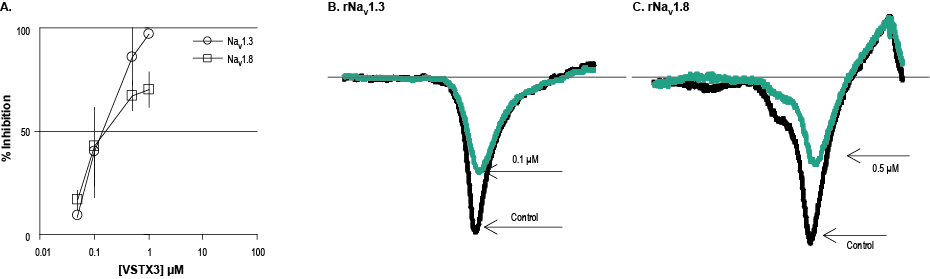 Alomone Labs VSTX3 inhibits rNaV1.3 and rNaV1.8 channels heterologously expressed in HEK293 and ND7-23 cells, respectively.A. Dose-response of NaV channels inhibition by VSTX3 (#STT-350). The inhibition was measured in 3-5 cells for each dose in rat NaV1.3 channels expressed in HEK293 cells (open circles) and 3-5 cells for rat NaV1.8 expressed in ND7-23 cells (open square) in the presence of 600 nM Tetrodotoxin citrate (#T-550). B. Example of superimposed current traces of rat NaV1.3 channel activity before (black) and during (green) application of 0.1 µM VSTX3. Holding potential was -100 mV and currents were stimulated every 10 seconds by a voltage ramp of 40 msec from holding potential to +60 mV. C. Example of superimposed current traces of rat NaV1.8 channel activity before (black) and during (green) application of 0.5 µM VSTX3, both in the presence of 600 nM Tetrodotoxin (with citrate). The same voltage protocol was used as in graph B.
Alomone Labs VSTX3 inhibits rNaV1.3 and rNaV1.8 channels heterologously expressed in HEK293 and ND7-23 cells, respectively.A. Dose-response of NaV channels inhibition by VSTX3 (#STT-350). The inhibition was measured in 3-5 cells for each dose in rat NaV1.3 channels expressed in HEK293 cells (open circles) and 3-5 cells for rat NaV1.8 expressed in ND7-23 cells (open square) in the presence of 600 nM Tetrodotoxin citrate (#T-550). B. Example of superimposed current traces of rat NaV1.3 channel activity before (black) and during (green) application of 0.1 µM VSTX3. Holding potential was -100 mV and currents were stimulated every 10 seconds by a voltage ramp of 40 msec from holding potential to +60 mV. C. Example of superimposed current traces of rat NaV1.8 channel activity before (black) and during (green) application of 0.5 µM VSTX3, both in the presence of 600 nM Tetrodotoxin (with citrate). The same voltage protocol was used as in graph B.
- Ruta, V. and MacKinnon, R. (2004) Biochemistry 43, 10071.
- Meir, A. et al. (2011) Novel peptides isolated from spider venom, and uses thereof. U.S. Patent Application Publication # US 2011/0065647 A1.
- Sheets, P.L. et al. (2008) J. Pharmacol. Exp. Ther. 326, 89.
- Zimmermann, K. et al. (2007) Nature 447, 855.
- Amir, E. et al. (2006) J. Pain 7, S1.
VSTX3 was originally isolated from the Grammostola spatulata spider venom1 and is also isolated from the Phrixotrichus auratus spider venom2.
VSTX3 was first isolated and shown to bind through its affinity to the voltage-sensor domain of KvAP channels1.
VSTX3 also inhibits voltage-gated rat NaV1.3, NaV1.7 and NaV1.8 Na+ channels2.
Voltage-gated sodium channels (VGSC, NaV) play a critical role in excitability of nociceptors (pain-sensing neurons). The peripheral-specific sodium channels NaV1.7, NaV1.8 and NaV1.9 are particularly important in the pathophysiology of different pain syndromes and hence, thought to be potential targets for pain therapeutics3,4.
The expression and functional properties of NaV channels in peripheral sensory neurons can be dynamically regulated following axonal injury or peripheral inflammation5.
VSTX3 shows high homology to Ceratotoxin-1 (75%), Ceratotoxin-2 (#STC-100) (77%) and Pterinotoxin-1 (#STT-100) (83%).
VSTX3 (#STT-350) is a highly pure, synthetic, and biologically active peptide toxin.

