Our brains are an intricate web of connections that form and reshape through development. And now, researchers have shed new light on a key mechanism in postnatal brain development that reveals some fascinating insights into the GluN3A NMDA receptor (NMDAR) subunit and its role in tuning NMDAR synaptic trafficking and content.
The Developmental Switch in NMDAR Subunits
During early development, the brain undergoes a remarkable transformation marked by the continuous creation and removal of synapses. This initial stage of flux is later refined through sensory input and neuronal activity, leading to the stabilization and strengthening of specific synaptic connections. The process is vital as it forms precise neuronal circuits from initially redundant connections – a critical step in fine-tuning cognitive functions and behaviors.
At the heart of this developmental journey is the shift in NMDAR subtypes, specifically the developmental switch from GluN2B-containing NMDARs to GluN2A-containing ones. This transition influences the biophysical and pharmacological properties of NMDARs, affecting the receptors’ signaling, localization, and temporal integration of synaptic inputs. Moreover, GluN3A subunits play a distinctive role in synapse stabilization and maturation, particularly during postnatal periods. Alterations in GluN3A expression during development can significantly impact synapse maturation, memory consolidation, and learning.
However, we still don’t fully understand the mechanisms governing the dynamics and diffusion of NMDARs between the synaptic and extrasynaptic compartments. Specifically, the way GluN3A subunits influence these dynamics, favoring an immature NMDAR phenotype at synapses, hasn’t been extensively studied.
To delve into this gap, the authors of this new article planned to track surface GluN3A-NMDARs in live hippocampal neurons and evaluate the impact of GluN3A subunit levels on synaptic NMDAR surface dynamics. They used a combination of single nanoparticle imaging, immunohistochemical, electrophysiological, and biochemical approaches to unveil the dynamics of GluN3A-NMDARs and their influence on the synaptic content of NMDARs in developing neurons.
The GluN3A Subunit: A Key Player
The team discovered that GluN3A-NMDARs are more diffusive and loosely anchored when compared to GluN2-NMDARs. GluN3A-NMDARs were shown to diffuse at the surface of hippocampal neurons depending on their activity. Moreover, GluN3A subunits controlled the dynamics and localization of GluN2A-NMDARs, but not GluN2B-NMDARs. Interestingly, the physical immobilization of GluN3A-NMDARs influenced GluN2A-NMDAR surface dynamics. And during early postnatal development, the GluN3A subunit guided GluN2A/GluN2B synaptic content.
Dynamics of Synaptic NMDARs
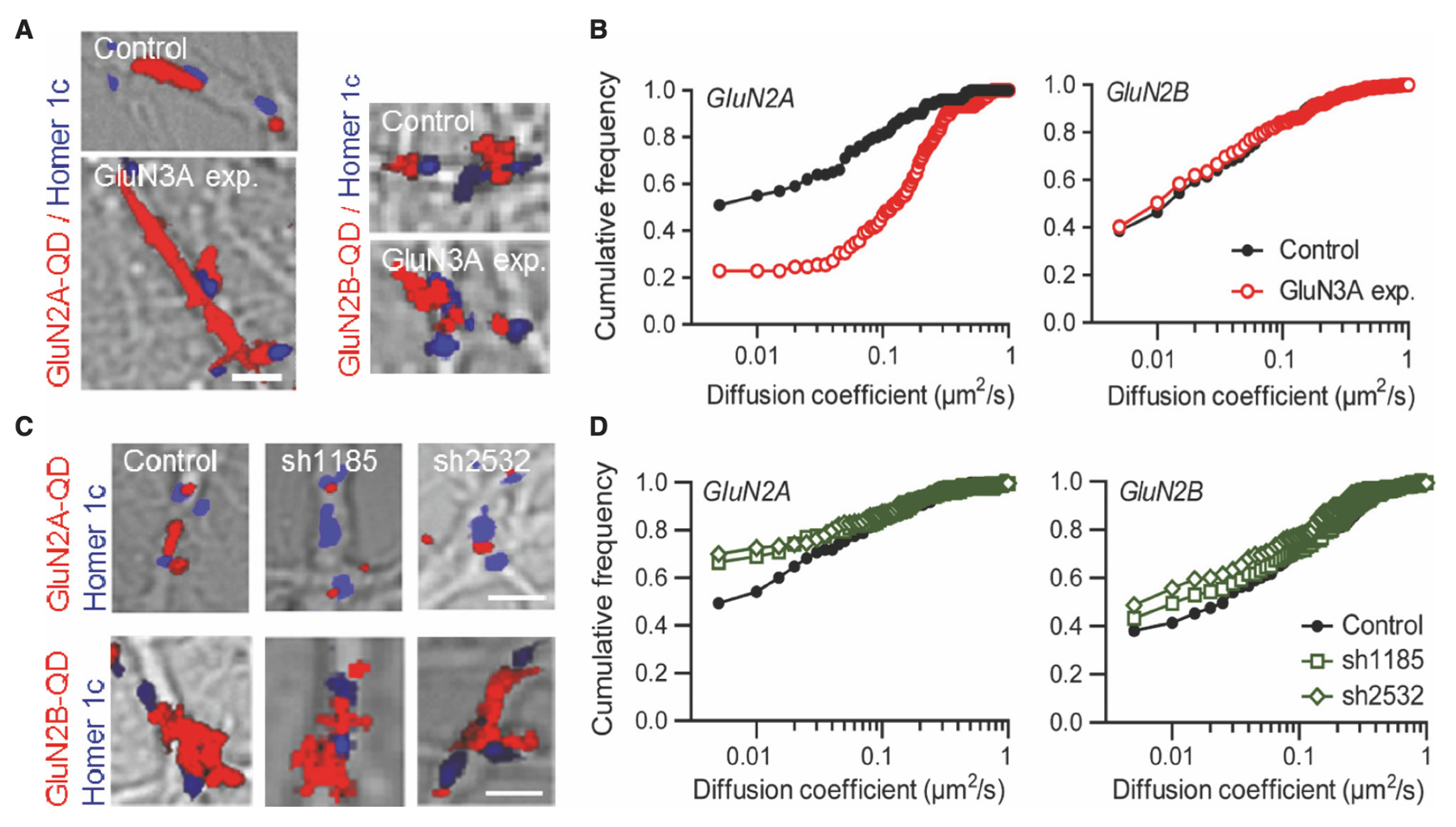
The researchers began their investigation by looking at GluN3A-NMDARs in cultured hippocampal neurons using a single-nanoparticle (quantum dot [QD]) tracking approach. They discovered that the synaptic number of NMDARs relies on a balance between receptors entering and exiting the postsynaptic region. Various interactions within the cellular structure influence the equilibrium of this dynamic, which depends on receptor subunit composition. For example, the surface diffusion of GluN2A-NMDARs is lower than that of GluN2B-NMDARs, likely due to differing anchoring mechanisms (Figure 2). To use QD tracking of GluN2A and GluN2B, the team incubated hippocampal neurons with antibodies against the N-terminal extracellular domain of GluN2A (Alomone Labs #AGC-002) epitope corresponding to residues 41–53 of GluN2A subunit) or GluN2B subunits (Alomone Labs #AGC-003) epitope corresponding to residues 323–337 of GluN2B subunit). This was followed by incubation with QD 655 donkey anti-mouse or rabbit IgG. The results showed that GluN3A subunits influence the positioning of GluN2A-NMDARs within the postsynaptic compartment by modifying both confinement and dwell times.
GluN3A Subunit Regulates the Surface Diffusion and Synaptic Retention of GluN2A- but not GluN2B-NMDARs
The Role of GluN3A Subunits in Synapse Maturation
The team’s data also indicate that the developmental decrease in GluN3A subunit expression aligns with the progressive incorporation of GluN2A-NMDARs to postsynaptic sites. The researchers propose a model in which GluN3A subunits ‘destabilize’ GluN2A-NMDARs at maturing synapses, thereby delaying their stabilization. Furthermore, GluN3A-NMDARs are affected by neuronal activity, which suggests a role in driving synaptic maturation.
EphB2R and GluN2A-NMDAR Signaling
It also seems clear that the transmembrane EphB2 receptor (EphB2R) plays a role in the maturation of GluN2-NMDAR signaling at glutamate synapses. The interaction between NMDARs and EphB2R is altered by knocking out the GluN3A subunit, but the study found that the destabilization of GluN2A-NMDARs by GluN3A is independent of interaction with PSD95. This discovery also suggests that the destabilization by GluN3A restricts the synaptic GluN2B-to-GluN2A switch to later stages of development, allowing GluN2B-NMDAR signaling predominance during critical developmental phases.
Impact on Neurodevelopment and Disorders
Finally, this research has described a mechanism in which GluN3A subunits prevent the stabilization of GluN2A-NMDARs at maturing synapses, favoring a lower GluN2A/2B synaptic ratio. This appears to serve as a regulatory mechanism to control the maturation of glutamatergic synapses, aligning with an evolutionary process critical for proper mammalian brain development. Furthermore, altered GluN3A expression has been found in various neurodevelopmental and psychiatric disorders, suggesting that changes in GluN3A expression may restrict synaptic plasticity and maturation, resulting in dysfunctional neural networks.
Bridging to Broader Perspectives
This study uncovers a unique mechanism in which GluN3A subunits prevent the premature stabilization of GluN2A-NMDARs. It adds another layer to our understanding of how synaptic maturation is coupled to experience and how developmentally restricted GluN3A expression may be critical for the proper evolution of mammalian brains. Alterations in this expression may lead to neurological and psychiatric disorders by restricting the temporal windows for synaptic maturation, rendering the in-depth understanding of NMDARs a valuable pursuit.
Antibodies
- Anti-NMDAR2A (GluN2A) (extracellular) Antibody (#AGC-002)
- Anti-NMDAR2A (GluN2A) (extracellular)-ATTO Fluor-488 Antibody (#AGC-002-AG)
- Anti-NMDAR2B (GluN2B) (extracellular) Antibody (#AGC-003)
- Anti-GluR3 (GluA3) (extracellular) Antibody (#AGC-010)
- Anti-PSD-95 Antibody (#APZ-009)
Pharmacological Tools
Blockers/Antagonists: small molecules
- MPX-004 (#M-280)
- CGP-37849 (#C-325)
- DL-AP7 (#D-200)
- Ifenprodil (+)-tartrate salt (hemitartrate salt) (#I-105)
- Ro 8-4304 hydrochloride (#R-165)
- TCN 201 (#T-190)
Activators/Agonists: small molecules
Explorer Kits

