Within the complex biology of cancer cells lies a rarely discussed, yet critical component: nerve fibers. Recent findings explain how melanoma exploits nerve fibers for its survival. Melanomas, it seems, harness the power of pain to compromise our body’s defense mechanisms.
Melanomas & Nerve Interactions
Cancer’s rich network of nerve fibers is one of the less common contributors to its pathogenesis. In prostate cancer, for example, neural progenitor cells can ultimately contribute to the development and dissemination of tumors. While the embedding of nerve fibers within tumors isn’t new, the consequences of this interaction, especially with pain-signaling sensory neurons (nociceptors), have remained elusive. In this incredible collaborative study, scientists have shown that when melanoma cells interact with these nociceptors, they not only promote the growth and activity of these nerves but also ramp up their sensitivity to painful stimuli.
Sensitizing the Pain Detectors
Melanomas actively influence the behavior of neighboring nociceptors, making them more reactive. The researchers were able to demonstrate that nociceptors, when cultured in the presence of melanoma cells, demonstrated enhanced responsiveness to pain-inducing stimuli, illustrating melanoma’s manipulative capabilities.
In their exploration into melanoma’s interplay with nerves, they noticed that even though neither human malignant cells nor immune cells expressed genes of neuronal origin (determined through RNA sequencing), their expression was seen in melanoma patient biopsies. Using Alomone Labs’ Anti-TRPV1 (ACC-030) antibody to identify TRPV1+ neurons, they discovered that these neurons were associated with the perception of pain and showed around a twofold increase in melanomas when compared to adjacent healthy tissue. Interestingly, the number of tumor-infiltrating lymphocytes (TILs) was found to correlate with the increase in these pain receptors (Figure 1).
TRPV1+ neurons innervate patient melanomas
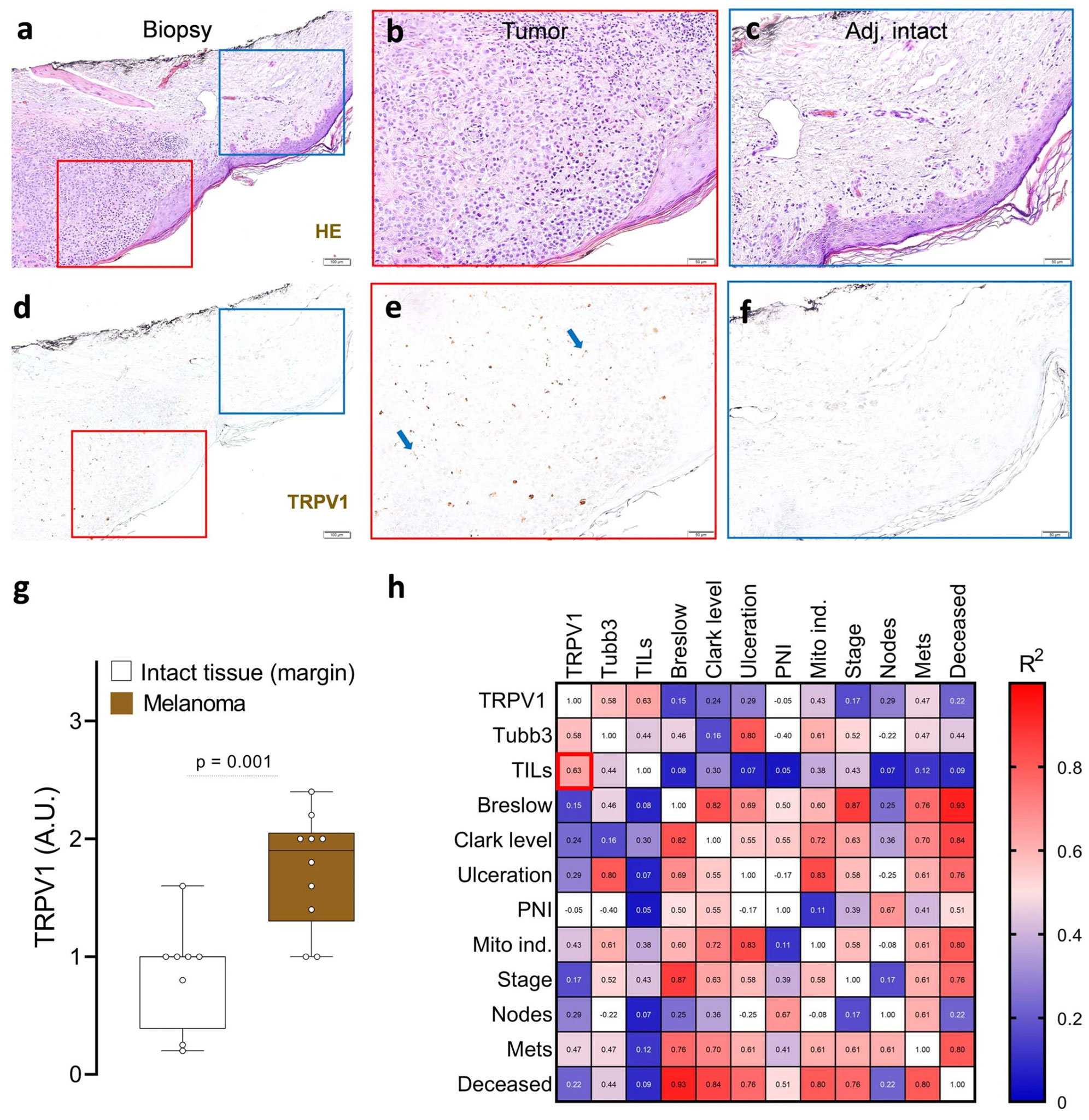
To delve deeper into this, GFP-expressing melanoma cells were introduced into specific mice breeds, revealing a significant presence of NaV1.8+ nociceptor neurons around and within the tumor. Additional in vitro studies showed that when TRPV1+ nociceptors and melanoma cells are cultured together, the nociceptors actively grow toward the melanoma cells. This behavior is akin to cancer’s neoangiogenesis, in which new blood vessels form to supply nutrients to tumors.
It would seem that melanomas have the ability to alter the sensitivity of the nociceptors. Tests involving noxious ligands revealed that melanoma cells increase the responsiveness of these pain receptors, resulting in heightened nociceptor sensitivity. And when DRG neurons were co-cultured with melanoma cells, there was an active release of calcitonin gene-related peptide (CGRP), an immunomodulatory neuropeptide, in the medium. Gene expression analysis confirmed that genes related to pain and pain modulation showed significant changes upon exposure to melanoma cells.
The Role of SLPI in Nociceptor Sensitization
To understand the mechanism by which melanoma sensitizes the nociceptors, a co-culture system mimicking the melanoma environment was developed. A significant finding was the overexpression of the secretory leukocyte protease inhibitor (SLPI) in melanoma cells when co-cultured with DRG neurons or specific cytotoxic T cells. SLPI, beyond its known functions, was found to activate DRG neurons, particularly the nociceptor neurons. This activation led to the release of CGRP from these neurons, shedding light on the pathways melanomas might use to heighten pain sensitivity. In vivo tests further established that SLPI does indeed cause heightened pain sensitivity when introduced to mice.
The researchers also found that melanoma-secreted SLPI acted on nociceptors to initiate calcium influx, neuropeptide release, and thermal hypersensitivity. This suggests that these pain receptors can detect and potentially react to the presence of malignant cells. An intriguing observation made was the positive correlation between heightened sensitivity to thermal pain and the increase in specific immune cells within the tumors.
The Role of CGRP: A Double-Edged Sword
One of the key players in this malignant alliance is the neuropeptide CGRP. Produced in abundance by these hypersensitive nociceptors, CGRP has an insidious role: it directly contributes to the exhaustion of CD8+ T cells, our immune system’s primary defense against melanomas. Why is this concerning? T cells, especially the cytotoxic CD8+ variety, are fully equipped to eliminate threats such as cancer cells. But CGRP appears to drain them of their vigor, impairing their ability to fend off the melanoma invasion.
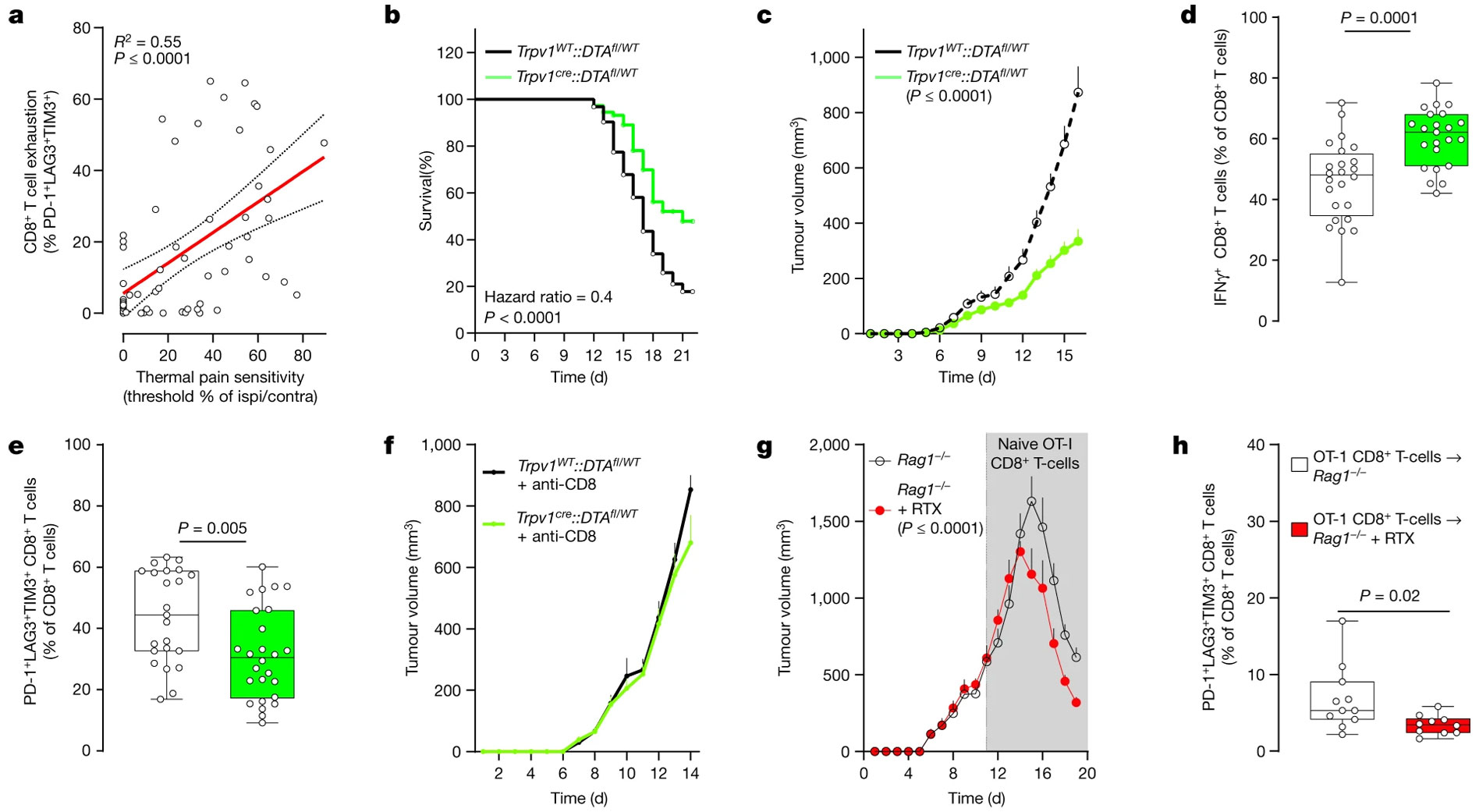
Using a mouse model of triple-negative melanoma, B16F10-mCherry-OVA cells were introduced into eight-week-old mice. Findings indicated that in the absence of nociceptors, not only was the lifespan of the mice extended by approximately 2.5-fold, but the tumor growth was also markedly reduced. Significantly, this reduction in tumor growth was coupled with an augmentation in the cytotoxic tumor-infiltrating CD8+ T cells. Conversely, in mice that had nociceptors ablated via an injection of Resiniferatoxin (R-400), there was a noted decrease in the proportion of PD-1+LAG3+TIM3+ CD8+ T cells (Figure 2).
The data paints a compelling picture, implying that nociceptor neurons play a pivotal role in driving the intratumoral population of exhausted T cells. This hypothesis is further supported by evidence showing thermal hypersensitivity manifesting days before any discernible exhaustion of intratumoral CD8+ T cells. Notably, this pain hypersensitivity precedes any measurable tumor growth.
Cell exhaustion is important because exhausted T cells lose their functional potency, including their ability to produce crucial immune molecules (IFNγ, TNF, and IL-2) and their proliferative capacity. These T cells also simultaneously increase their expression of exhaustion markers, such as PD-1, LAG3, and TIM3, rendering them less effective in eliminating cancer cells.
Genetic ablation of nociceptors safeguards anti-tumor immunity
To address potential compensatory changes due to the early ablation of neuronal subsets, neurons were silenced using botulinum neurotoxin A (BoNT/A). BoNT/A effectively reduced tumor growth without affecting the function of both tumor cells and CD8+ T cells. Its efficacy, however, seemed to be contingent on the presence of nociceptor neurons. In an alternative approach, the silencing efficacy of a known nociceptor-selective strategy was examined. QX-314 was used to block large-pore ion channels, resulting in a long-lasting electrical blockade. Remarkably, QX-314 not only reduced melanoma growth but also preserved the integrity and functionality of intratumoral CD8+ T cells, reinforcing its potential therapeutic utility in the realm of cancer.
The Bigger Picture and Implications for Cancer Treatment
The research described here has explored how, in melanoma, the presence of nociceptor neurons increases the production of CGRP, which in turn affects the capability of CD8+ T cells in the tumor vicinity. The increased CGRP levels correlate with a higher expression of immune checkpoint receptors in CD8+ T cells, leading to their diminished capacity to fight the tumor.
This meeting of nervous and immune systems has profound implications for melanoma research and cancer therapy. A deeper understanding of the role of CGRP in reducing CD8+ T cells’ ability to tackle cancer is paramount. We now know that when nociceptor neuron activity is inhibited, the functionality of CD8+ T cells is preserved, highlighting the significant role of CGRP in T cell exhaustion. Furthermore, the RAMP1 antagonist, BIBN4096, showed promise in reducing tumor growth and the frequency of exhausted CD8+ T cells, suggesting that the drug could potentially target the neuro-immune interactions that favor tumor progression.
Some of the data from this study point toward a potential breakthrough in melanoma research and cancer therapy. The authors speculate that by interrupting the CGRP–RAMP1 axis, it might be possible to rejuvenate the effectiveness of CD8+ T cells, enhancing the body’s innate ability to fight cancers, such as melanoma. This not only opens doors for developing drugs targeting the CGRP–RAMP1 axis, but also offers hope for patients who are resistant to current immunotherapies, such as immune checkpoint inhibitors. By tackling the neuro-immune links that foster a pro-cancer environment, new therapeutic strategies can be envisioned that synergize the power of both the nervous and immune systems against malignancies.
This intertwining of melanoma cells with pain-initiating neurons has contributed to our understanding of progression. While the pain-cancer meeting might seem counterintuitive or unlikely at first, understanding its dynamics could very well offer new therapeutic strategies in the field of melanoma research.
Antibodies
- Anti-TRPV1 (VR1) Antibody (#ACC-030)
- Anti-Rat TRPV1 (VR1) (extracellular) Antibody (#ACC-029). This antibody recognizes a different epitope and thus can also be used as a control for detecting TRPV1. This antibody should be used with rat samples only.
- Guinea pig Anti-TRPV1 (VR1) Antibody (#ACC-030-GP). This antibody is raised in guinea pig and can be used in multiplex staining studies in conjunction with any of our antibodies raised in rabbit. It has been raised against the same epitope as that of #ACC-030.
- Anti-Rat-TRPV1 (VR1) (extracellular)-ATTO Fluor-488 Antibody (#ACC-029-AG). A fluorescent labeled primary antibody. It can be used in multiplex staining studies in conjunction with any of our antibodies raised in rabbit. This antibody should be used with rat samples only.
- Anti-Human TRPV1 (extracellular)-FITC Antibody (#ACC-334-F). This FITC-conjugated antibody can be used to detect TRPV1 in live cell flow cytometry.
- Anti-Human TRPV1 (extracellular)-APC Antibody (#ACC-334-APC). This APC-conjugated antibody can be used to detect TRPV1 in live cell flow cytometry.
Pharmacological Tools
- Resiniferatoxin (#R-400). A Potent and Selective Activator of TRPV1 Channel.
- 5′-Iodoresiniferatoxin (#I-800)
- A 784168 (#A-345)
- 6′-Iodoresiniferatoxin (#I-805)
- Capsaicin (#C-125)
Explorer Kits
Research Packs
- TRPV1 Channel Basic Research Pack (#ESB-300)
- TRPV1 Channel Premium Research Pack (#ESP-300)
- TRPV1 Channel Deluxe Research Pack (#ESD-300)

